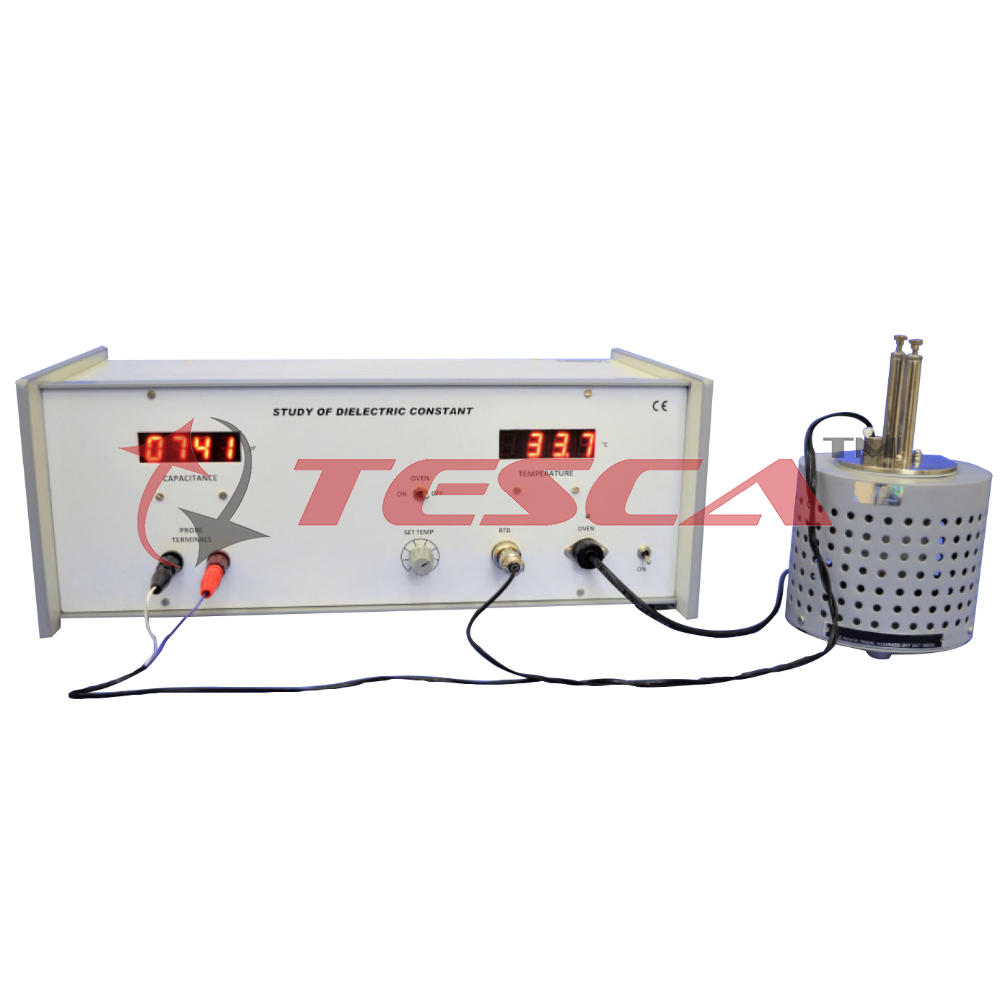
Study of Dielectric Constant & Curie Temperature of Ferroelectric Ceramics
Order Code: 55525
Category: Physics Trainers
Dielectric or electrical insulating material are understood as the material in which electrostic field can persist for long times. Layers of such substance are commonly inserted into capacitors to improve their performance, and the term dielectric re...
SPECIFICATION
Dielectric or electrical insulating material are understood as the material in which electrostic field can persist for long times. Layers of such substance are commonly inserted into capacitors to improve their performance, and the term dielectric refers specifically to this application.
An electric field polarizes the molecules of dielectric producing concentrations of charge on its surface that create an electric field opposed (antiparallel) to that of capacitor. This reduces the electric potential. Considered in reverse, this means that, with a dielectric, a given electric potential causes the capacitor to accumulate a larger charge.
Applications
Beside the common and well known application of capacitors in electrical and electronic circuits, the capacitors with an exposed and porous dielectric can be used to measure humidity in air.
A huge leap in the research on dielectric (ferroelectric materials) came in 1950’s, leading to the wide spread use of Barium Titanate (BaTiO 3-Perovskite Structure) based ceramics in capacitor applications and piezoelectric transducer devices. Since then, many other ferroelectric ceramics have been developed and utilized for variety of applications: various type of capacitors, non volatile memories in computers, etc.
Perovskite Structure
Perovskite is family name of a group of materials and the mineral name of calcium titanate (CaTiO 3) having a structure of the type ABO3 (Fig 1)
A practical advantage of perovskite structure is that many different cations can be substituted on both A and B sites without changing the over all structure. Even though two cations are compatible in solution, their behaviour can be radically different when apart from each other. Thus it is possible to manipulate material’s properties such as Curie temperature or dielectric constant with only a small substitution of given cation.
All ferroelectric material have a transition called the Curie point (TC) . At T>TC , the crystal does not exhibit ferroelectricity, while for T<TC it is ferroelectric. If there is more than one ferroelectric phase, the temperature at which the crystal transforms one phase to another is called transition temperature. Near the Curie temperature point or transition temperatures, the thermodynamic properties including dielectric, elastic, optical and thermal constants show an anomalous behavior.
Fig.2 shows the variation of dielectric constant (e) with temperature for Lanthanum doped Lead Zirconate Titanate ( PLZT) ceramic, which is cooled from its paraelectric cubic phase to ferroelectric rhombohedral phase.
- Temperature Range : Ambient to 200°C
- Display : 31/2 digit, 7 segment LED with
- autopolarity & decimal
- indication
- Resolution : 0.1°C
- Accuracy : ±0.5°C (typical)
- Stability : ±0.1°C
- Power : 150W
- Range : 50-6000 pf
- Resolution : 1pf
- Display : 3½ digit, 7 segment LED









 91-9829132777
91-9829132777