Planck's Constant by Photoelectric Effect

Order Code: 55528
Category: Physics Trainers
1. Determination of planck's constant and work function of materials by photoelectric effect It was observed as early as 1905 that most metals under influence of radiation, emit electrons. This phenomnon was termed as photoelectric e...
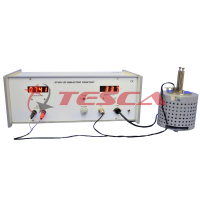
SPECIFICATION
1. Determination of planck's constant and work function of materials by photoelectric effect
It was observed as early as 1905 that most metals under influence of radiation, emit electrons. This phenomnon was termed as photoelectric emission. The detailed study of it has shown.
- That the emission process depends strongly on frequency of radiation.
- For each metal there exists a critical frequency such that light of lower frequency is unable to liberate electrons, while light of higher frequency always does.
- The emission of electron occurs within a very short time interval after arrival of the radiation and member of electrons is strictly proportional to the intensity of this radiation.
The experimental facts given above are among the strongest evidence that the electromagnetic field is quantified and the field consists of quanta of energy E= hv where v is the frequency of the radiation and h is the Planck's constant. These quanta are called photons.
Further it is assumed that electrons are bound inside the metal surface with an energy eF, where (I) is called work function. It then follows that if the frequency of the light is such that
hv > eF
it will be possible to eject photoelectron, while if hv<eF, it would be impossible. In the former case, the excess energy of quantum appears as kinetic energy of the electron, so that which is the famous photo electrons equation formulated by Einstein in 1905.
The energy of emitted photoelectrons can be measured by simple retarding potential techniques as is done in this experiment. When a retarding potential Vo is used to measure kinetic energy of electrons Ee, we have,
Ee= mv2=eVo or Vo= v-F
So when we plot a graph Vo as a function of n, the slope of the straight line yields h and the intercept of extrapolated point v=0 can give work function F.
2. To verify inverse square law of radiation using a photoelectric cell
If L is the luminous intensity of an electric lamp and E is the illuminanscence (intensity of illumination) at point r form it, then according to inverse square law.
if this light is allowed to fall on the cathode of a photo-electric cell, then the photo-electric current (I) would be proportional to E.
Hence a graph between zand I is a straight line, which verify the inverse square law of radiation.
The apparatus consist of the following :
- Photo sensitive device : Vacuum photo tube.
- Light source : Halogen tungsten lamp 12V/35W.
- Colour filters : 635nm, 570nm, 540nm, 500nm a 460nm.
- Accelerating voltage : Regulated Voltage Power Supply
- Output : ± 15 V continuously variable through multi-turn pot
- Display : 3 1/2 digit 7-segment LED
- Accuracy :±0.2%
- Current detecting unit : Digital Nanoammeter is a high stability low current measuring instrument
- Range : 10001.1A, 1001.1A, 10[tA a 1µA with
- 100% over ranging facility
- Resolution : 1 nA at l[tA range
- Display : 3 1/2 digit 7-segment LED
- Accuracy : ±0.2%
- Power requirement : 220V ± 10%, 50Hz.
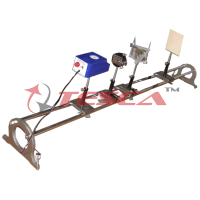
- Optical Bench : The light source can be moved along it to adjust the distance between light source and photo tube. Scale length is 400 mm. A draw tube is provided to install colour filters, a focus lense is fixed in the back end.
The set is complete in all respect, no additional accessory required.









 91-9829132777
91-9829132777